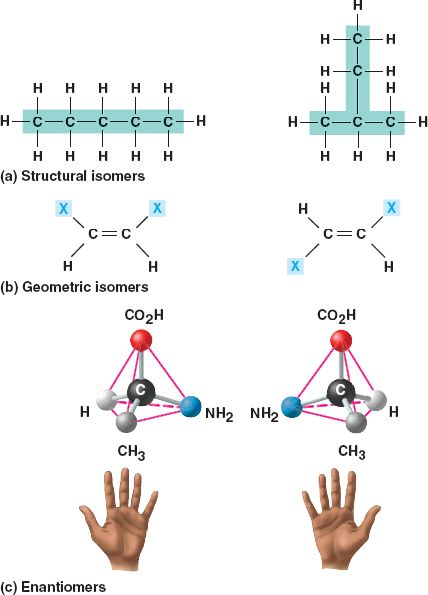
 Isomers are compounds with the same molecular formula but different structures and properties.
Isomers are compounds with the same molecular formula but different structures and properties.
- Structural isomers
differ in the arrangement of their covalent
bonds.
- Geometric isomers differ in their spatial arrangements around a double
bond.
- Enantiomers (stereo isomers)
are mirror images of each other and contain an asymmetric
carbon.
Review: 

 Isomers are compounds with the same molecular formula but different structures and properties.
Isomers are compounds with the same molecular formula but different structures and properties.
